Lentivirus
Curia offers a comprehensive suite of lentivirus services, from vector engineering and production through analytics. We utilize the third generation four plasmid system and the HEK293 cell line for the production of lentiviruses. Custom vector design and development for the production of a variety of molecules, including CARs, is available.
Key Features of Lentivirus Platform
– Used for CAR-T and cell line engineering
– 3rd generation, four plasmid system
– Produced in HEK293-based system
Adherent & Suspension
– Standard titer: 107 to 109 IFU/mL
– Pseudotyping
CAR Vector Selections Available
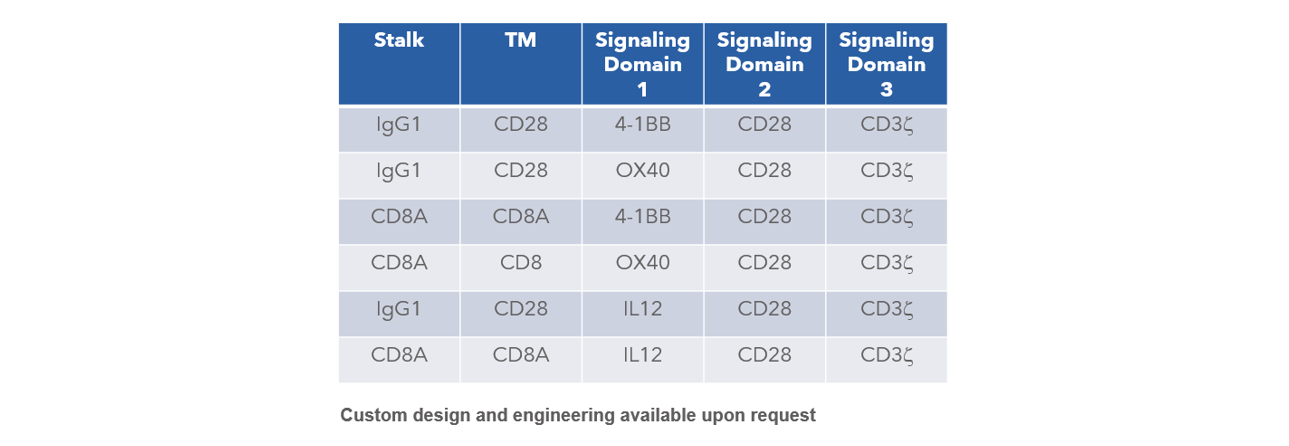
In addition to lentivirus production, Curia also offers cell engineering services, including CAR-T.
Curia’s Custom Lentivirus Production Services
| Vector Engineering | Upstream | Downstream | Analytics |
|---|---|---|---|
| – Vector design – Gene synthesis – Genetic cloning – Plasmid production |
– Adherent culture – Suspension culture – Wave bioreactors (300L) |
– Homogenization – Chromatography – Affinity, IEX, etc. – Ultracentrifugation |
– Infectious titer – Purity (PAGE) – Full: Empty Capsid – Bioburden & more |

